CBSE Class 10 Chapter 1
Chemical Reactions and Equations - Notes
Introduction to Chemical Equations:
- Chemical equations are a way to represent chemical reactions.
- They show the transformation of reactants into products using an arrow (→).
- The law of conservation of mass states that mass is conserved in chemical reactions.
Skeletal Chemical Equation:
- An unbalanced equation is often referred to as a "skeletal chemical equation".
- It's a basic representation of the reaction without the proper balance of atoms.
Balanced Equation:
- If the number of atoms of each element is the same on both sides, it means the equation is balanced.
- A balanced equation follows the law of conservation of mass, ensuring that mass is conserved in the reaction.
Unbalanced Equation:
- If the number of atoms of any element is different on the LHS and RHS, the equation is unbalanced.
- In an unbalanced equation, the total mass is not the same on both sides.
Word-Equation:
- A word-equation describes a chemical reaction using words.
- For example-
Magnesium +
Oxygen → Magnesium oxide
(Reactants) (Product)
- In this equation, the substances that undergo a chemical change are:
- Reactants: Magnesium and oxygen
- Product: Magnesium oxide
- A word-equation shows change of reactants to products through an arrow placed between them.
- Reactants are written on the left-hand side (LHS) of the equation with a plus sign (+) between them.
- Similarly, products are placed on the right-hand side (RHS) of the equation with a plus sign (+) between them.
- The arrowhead, pointing rightwards (→), indicates the direction of the chemical reaction. It shows that reactants are changing into products.
Chemical Equations by using chemical formulae:
- Chemical equations can be made more concise by using chemical formulae.
- For example-
Mg + O2
→ MgO
Balancing Chemical Equations:
Counting Atoms:
- To balance a chemical equation, you first count the number of atoms of each element on both sides of the arrow.
- This means you check how many atoms of each element are on the left-hand side (LHS) and the right-hand side (RHS) of the equation.
Steps for Balancing:
Let’s take a Skeletal Chemical Equation –
Fe2
+ H2O → Fe3O4 + H2
Step I: Draw boxes around formulae.
Fe2 + H2O → Fe3O4
+ H2
Step II: List the number of atoms of different elements on both
sides.
|
Element |
Number of Atoms in reactants (LHS) |
Number of Atoms in Product (RHS) |
|
Fe |
1 |
3 |
|
H |
2 |
2 |
|
O |
1 |
4 |
Step III: Start with the compound containing the maximum number of
atoms. It may be a reactant or a product. In that compound, select the element which
has the maximum number of atoms. Here, Fe3O4 is
compound and element is O.
Step IV: Equalize one element at a time, without altering
formulae.
(i) To balance Oxygen Atom-
|
Atom of Oxygen |
In reactant |
In products |
|
In Initial
stage |
1 (in H2O) |
4 (in Fe3O4) |
|
To balance |
1×4 |
4 |
Remember we should not be altering the formulae of compound or
elements which involved in reaction.
Now make partially balanced equation:
Fe2 + 4 H2O → Fe3O4 + H2
(ii) Balancing H atom:
|
Atom of Hydrogen |
In reactant |
In products |
|
In Initial
stage |
8 (in 4H2O) |
4 (in H2) |
|
To balance |
8 |
2×4 |
Now make partially balanced equation:
Fe2 + 4 H2O → Fe3O4 + 4 H2
(iii) Balancing Fe atom:
|
Atom of Iron |
In reactant |
In products |
|
In Initial
stage |
1 (in Fe) |
3 (in Fe3O4) |
|
To balance |
1×3 |
3 |
Now make final balanced equation:
3 Fe2 + 4 H2O → Fe3O4
+ 4 H2
Step V: Ensure equal numbers of atoms on both sides.
3 Fe2
+ 4 H2O → Fe3O4
+ 4 H2
This method is called hit-and-trial method because we make trials
to balance the equation by using the smallest whole number coefficient.
Step VI: Including Physical States and Conditions:
Physical states for example (g) for gas, (l) for liquid, (aq) for aqueous
solution, (s) for solid, gas produced in the reaction (↑), precipitate formed
in the reaction (↓) can be added for clarity.
Reaction conditions like- temperature, pressure, catalyst can be
indicated above or below arrow sign.
3 Fe2 (s) + 4 H2O (g) → Fe3O4 (s) + 4 H2 (g)
Types of Chemical Reactions:
Combination Reactions:
- Two or more reactants combine to form a single product.
A + B → AB
Examples: (i)
CaO(s) + H2O(l) → Ca(OH)2(aq) + Heat
(Quick
lime) (Slaked
lime)
In this reaction, calcium oxide reacts with water and produced
slaked lime (calcium hydroxide) and release heat.
Note : Calcium hydroxide is used for whitewashing on walls. It
react slowly with carbon dioxide and make a thin layer of calcium carbonate on
wall and give a shiny finish after two or three day.
Ca(OH)2(aq)
+ CO2(g) → CaCO3(s) + H2O(l)
(Calcium hydroxide) (Calcium carbonate)
Chemical formula for calcium carbonate and marble are same CaCO3
(ii) Burning of coal
C(s) + O2(g)
→ CO2(g)
(iii) Formation of water from H2(g) and O2(g)
2H2(g)
+ O2(g) → 2H2O(l)
Exothermic chemical reactions:
- Reactions in which heat is released along with the formation of products are called exothermic chemical reactions.
Examples: (i) Burning of natural gas (Methane)
CH4(g)
+ 2O2 (g) → CO2 (g) + 2H2O (g) + Heat
Methane +
Oxygen → Carbon dioxide + water + Heat
(ii)
C6H12O6(aq)
+ 6O2(aq) → 6CO2(aq) + 6H2O(l) + energy
Food like- rice, potatoes and bread contain carbohydrates. These carbohydrates
are broken down to form glucose during digestion process. This glucose combines
with oxygen in the cells of our body and provides energy. This reaction process
called respiration,
(iii) The decomposition of vegetable matter into compost.
(iv) When an acid reacts with a base, it generates heat.
HCl + NaOH →
NaCl + H2O + heat
hydrochloric
acid + sodium hydroxide → salt + water + heat
Decomposition Reaction:
- A compound breaks down into simpler substances. It is the opposite of a combination reaction.
- There is one reactant compound that undergoes the breakdown.
- Decomposition reactions can be triggered by heat, light, electricity, or chemical reactions with other substances.
AB → A + B
Examples: (i) When calcium carbonate (as in limestone) is heated,
it decomposes into calcium oxide and carbon dioxide.
- Calcium oxide or Quick lime is used in Cement Industry.
- Thermal decomposition: When a decomposition reaction is carried out by heating, it is called thermal decomposition.
(ii) Ferrous sulphate crystals (FeSO4, 7H2O) lose water when heated and the colour of the crystals changes.
(iii) Heating of lead nitrate and emission of nitrogen dioxide- It is because the decomposition of silver chloride into silver and chlorine by light.
- Silver bromide also behaves like this.
- The above mention reactions are used in black and white photography.
(vii) Potassium chlorate decomposes upon heating to produce
potassium chloride and oxygen gas.
Endothermic Reactions:
- Reactions in which energy is absorbed are known as endothermic reactions. Above all reaction are endothermic because they need energy like- heat, sunlight, electric current.
Displacement Reaction or single replacement reaction:
- This is types of chemical reaction in which one element or ion in a compound is replaced by another element.
- Only one element or ion is replaced in a compound, while the other remains unchanged.
- The reaction occurs when a more reactive element displaces a less reactive element from a compound.
- The result of a displacement reaction is the formation of a new compound and the liberation of the displaced element.
Examples: (i) When we dip iron nail in copper sulphate solution, the
iron nail become brownish in colour and the blue colour of copper sulphate
solution become fades.
Fe(s) +
CuSO4(aq) → FeSO4(aq) + Cu(s)
Ferrous
+ copper sulphate → Ferrous sulphate + copper
- In this reaction, Iron has displaced another element, copper, from copper sulphate solution. Due to this displacement it is known as displacement reaction.
(ii)
Zn(s) +
CuSO4(aq) → ZnSO4(aq) + Cu(s)
Zink +
Copper Sulphate → Zink Sulphate + copper
- In this reaction, zinc (Zn) displaces copper (Cu) from copper sulfate (CuSO4), leading to the formation of zinc sulfate (ZnSO4) and the deposition of copper.
(iii)
Pb(s) +
CuCl2(aq) → PbCl2(aq) + Cu(s)
Lead + Copper
chloride → Lead chloride + copper
- In this reaction, Lead (Pb) displaces copper (Cu) from copper chloride (CuCl2), leading to the formation of Lead chloride (PbCl2) and the deposition of copper.
Double Displacement Reaction or double replacement reaction or
metathesis reaction:
- This is a type of chemical reaction that involves the exchange of ions between two compounds in aqueous solutions.
- Precipitate: A double displacement reaction leads to the formation of a solid substance called precipitate, which is insoluble in water.
- The end result of a double displacement reaction is the formation of two new compounds, one of which may be a precipitate.
Examples: (i)
Na2SO4(aq)
+ BaCl2(aq) → BaSO4(s) + 2NaCl(aq)
Sodium
sulphate + Barium chloride → Barium sulphate + Sodium chloride
- In this reaction, the white precipitate of BaSO4 is formed by the reaction of SO42- and Ba2+ Ions. The other product formed is sodium chloride which remains in the solution.
(ii) The reaction between silver nitrate (AgNO3) and
sodium chloride (NaCl), which produces silver chloride (AgCl) as a white solid
precipitate.
AgNO3(aq)
+ NaCl(aq) → AgCl(s) + NaNO3(aq)
Silver
nitrate + Sodium chloride → Silver chloride + Sodium Nitrate
(iii) The reaction between hydrochloric acid (HCl) and sodium
hydroxide (NaOH), forming water and sodium chloride (NaCl).
HCl(aq)
+ NaOH(aq) → H2O(l) + NaCl(aq)
Hydrochloric
Acid + Sodium hydroxide → Water + Sodium chloride
(iv) The reaction between hydrochloric acid (HCl) and sodium
sulfide (Na2S) produces hydrogen sulfide (H2S) gas.
2HCl(aq)
+ Na2S(aq) → H2S(g) + 2NaCl(aq)
Oxidation and Reduction or redox reactions:
Oxidation:
- Oxidation is a process in which a substance loses electrons (the number of positive charges on the atom). So, its oxidation state increased.
- It's like a substance sharing its electrons with others or losing its own.
- Oxidation often involves the addition of oxygen or the removal of hydrogen.
Reduction:
- Reduction is a process in which a substance gains electrons (the number of positive charges on the atom). Reduction is the opposite of oxidation. So, its oxidation state decreased.
- It's like a substance accepting electrons from others.
- Reduction usually involves the removal of oxygen or the addition of hydrogen.
Redox Reactions:
- Redox reactions are chemical reactions in which both oxidation and reduction occur simultaneously. One substance is oxidized (loses electrons) and another is reduced (gains electrons).
- The transfer of electrons from the oxidized substance to the reduced substance is a key feature of redox reactions.
- Redox reactions are essential in various processes, including combustion, respiration, corrosion, and electrochemical reactions (e.g., batteries).
Examples:
- When we heat copper powder in presence of oxygen it became copper oxide with black colour because oxygen added with copper and form copper oxide. In this reaction, copper gain oxygen or electrons and is being oxidized.
- When we pass hydrogen on heated copper oxide it became brown and copper is obtained.
- In this reaction, copper oxide losing oxygen and is being reduced.
- These types of reaction called redox reactions.
(ii) ZnO + C → Zn +
CO
- In this reaction, Carbon (C) is oxidized to Carbon mono oxide (CO) and ZnO is reduced to Zn.
(iii) MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
- In this reaction, HCl is oxidized to Cl2 and MnO2 is reduced to MnCl2.
The Effects of Oxidation Reactions in everyday life:
Corrosion:
- Corrosion is a natural process that occurs when metals react with their environment, usually in the presence of moisture, oxygen or other chemicals. This reaction makes the metal slowly break down, bit by bit.
- The oxidation of a metal takes place in this. In this process, the metal atoms lose electrons and transform into metal ions.
Rancidity:


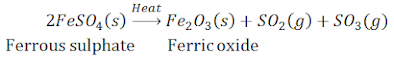






No comments:
Post a Comment
If you have any questions or uncertainties, please don't hesitate to ask.