CBSE Class 10 Science Chapter 2 Acids, Bases and Salts - Notes
Remind terms which learn in previous class:
Matter
- Matter is the substance that makes up everything in the universe.
- It is anything that has mass and takes up space.
- Matter is the physical substance of the universe which we can see, touch, taste and smell, as well as things that are too small to be seen without the aid of powerful microscopes, such as atoms and molecules.
- Matter is made up of atoms and molecules, which are the building blocks of all substances. These atoms and molecules interact with each other through various forces, such as electromagnetic forces, to create the properties and behaviors of different types of matter.
States of matter:
On the basis of States:
Solid:
- In this state, matter has a definite shape and volume.
- Particles in a solid are closely packed and held together by strong forces, making them vibrate in place. Examples- rocks, metals and wood.
Liquid:
- In this state, matter has a definite volume but takes the shape of its container.
- Particles in a liquid are not as closely packed as in a solid and they have more freedom to move past each other. Examples- water, oil and milk.
Gas:
- In this state, matter takes both the shape and volume of its container.
- Particles in a gas are widely spaced and move freely, filling the available space. Examples- air, oxygen and carbon dioxide.
Plasma:
- Plasma is a less common state of matter, typically occurring at very high temperatures.
- It consists of ionized particles and is often found in stars, lightning and some experimental situations.
Bose-Einstein Condensate (BEC):
- BEC is an exotic state of matter that occurs at extremely low temperatures, close to absolute zero.
- In this state, atoms lose their individual identity and behave as a single quantum entity.
On the basis of Composition:
Element:
- An element is a pure substance composed of only one type of atom.
- Elements cannot be broken down into simpler substances by chemical means.
- They are listed on the periodic table.
- Examples: oxygen (O), gold (Au) and carbon (C).
Compound:
- A compound is a pure substance composed of two or more different elements chemically combined in fixed proportions.
- Compounds can be broken down into their constituent elements through chemical reactions.
- Examples: water (H2O), carbon dioxide (CO2) and table salt (NaCl).
Mixtures:
Homogeneous Mixture:
- In this mixture, the components are uniformly distributed throughout the mixture and it appears visually the same throughout.
- Homogeneous mixtures are also known as solutions.
- Examples: saltwater, sugar dissolved in water and air.
Heterogeneous Mixture:
- In this mixture, the components are not uniformly distributed and we can often see distinct phases or regions within the mixture.
- Examples: Salad with different ingredients, a mixture of oil and water and a bowl of cereal with milk.
Colloids:
- Colloids are a specific type of mixture in which small particles or droplets of one substance are dispersed throughout another substance.
- Colloids can display some interesting properties, such as the Tyndall effect (scattering of light).
- Examples: milk, mayonnaise and fog.
Suspensions:
- Suspensions are mixtures in which larger particles are suspended in a liquid or gas, but they tend to settle over time.
- The particles in a suspension are typically much larger than those in a colloid.
- Examples: muddy water, orange juice with pulp and certain medical suspensions.
Types of compounds:
Covalent Compounds (Molecular
Compounds):
- Covalent compounds are formed when atoms share electrons to achieve a stable electron configuration.
- Covalent bonds involve the sharing of electrons between two or more non-metal atoms.
- Covalent compounds are often found in the gas or liquid state at room temperature and pressure. They can also exist as solids.
- Examples: Water (H2O), carbon dioxide (CO2), methane (CH4) and ammonia (NH3)
Ionic Compounds (Salts):
- Ionic compounds are formed when electrons are transferred from one atom (usually a metal) to another atom (usually a non-metal).
- This results in the formation of positively charged ions (cations) and negatively charged ions (anions).
- These oppositely charged ions are attracted to each other due to electrostatic forces, forming ionic bonds.
- Ionic compounds are composed of a three-dimensional lattice structure of alternating cations and anions. They do not exist as discrete molecules.
- Examples: Common table salt, sodium chloride (NaCl), calcium chloride (CaCl2) and potassium sulfate (K2SO4)
Non-Ionisable and Ionisable Compounds:
Non-Ionizable Compounds (Nonelectrolytes):
- Non-ionizable compounds are substances that do not dissociate into ions when they are dissolved in water.
- They do not conduct electricity when in solution because they do not produce ions.
- Non-ionizable compounds are typically covalent compounds and their solutions do not conduct electricity.
- Examples: most of organic molecules, such as sugars (glucose), alcohols (e.g., ethanol) and gases (oxygen and nitrogen).
- Ionizable compounds are substances that can dissociate into ions (charged particles) when they are dissolved in water.
- They can conduct electricity when in solution because of the presence of ions that can move and carry an electric charge.
- Ionizable compounds are typically soluble in water to some extent and their solutions can conduct electricity. These solutions are called electrolyte solutions.
- Examples: acids, bases, and salts.
Acids:
Acids are ionizable
compounds that release hydrogen ions (H+) when dissolved in water.
Examples: hydrochloric acid (HCl), sulfuric acid (H2SO4)
and acetic acid (CH3COOH).
Bases:
Bases are ionizable
compounds that release hydroxide ions (OH—) when dissolved in water.
Examples: sodium hydroxide (NaOH) and potassium hydroxide (KOH).
Salts:
Salts are ionizable
compounds composed of positively charged cations and negatively charged anions.
When dissolved in water, salts dissociate into their constituent ions. Examples:
table salt sodium chloride (NaCl) dissociates into sodium ions (Na+)
and chloride ions (Cl—) in water.
The Chemical Properties of Acids and
Bases:
(i) Acid-Base Reactions:
- Acids react with bases to form water and a salt in a chemical process known as neutralization.
- This reaction involves the transfer of hydrogen ions (H+) from the acid to the base to form water.
- Its common form is : Base + Acid → Salt + Water
Example (i): The reaction between hydrochloric acid (strong acid) and sodium hydroxide (strong base), forms salt and water.
HCl (Strong acid) + NaOH (Strong base) → NaCl + H2O
hydrochloric acid + sodium hydroxide → salt + water
(ii) (a) Reaction of Metallic Oxides with Acids:
In this chemical reaction, the oxide
acts as a base and the acid donates hydrogen ions (H+). The general reaction form
can be represented as follows:
Metallic Oxide + Acid → Salt + Water
Example (i):
CuO + 2HCl → CuCl2 + H2O
Copper (II) oxide + hydrochloric acid → copper (II)
chloride + H2O
Example (ii):
ZnO + H2SO4 → ZnSO4 + H2O
zinc oxide + sulfuric acid → zinc sulfate + water
Example
(iii):
Fe2O3 + 6HNO3 →
2Fe(NO3)3 + 3H2O
Iron (III) oxide + nitric acid → iron (III) nitrate + water
Example (iv):
MgO + H2SO4 → MgSO4
+ H2O
Example (v):
Mg (OH)2 + 2HCl → MgCl2 + 2H2O
Note : In above examples, the metallic
oxide acts as a base by accepting hydrogen ions (H+) from the acid.
(b) Reaction of Non-Metallic Oxides
with Base:
- When non-metallic oxides react with bases they form salts or oxides and water.
- The specific products depend on the non-metallic oxide and the base involved in the reaction.
- The non-metallic oxides are acidic in nature.
Example (i):
SO2 + 2NaOH → Na2SO3
+ H2O
Sulpher dioxide + sodium hydroxide → sodium sulfite
+ water
Here SO2 is a non-metallic oxide and
NaOH is base.
Example (ii):
CO2 + Ca(OH)2 → CaCO3 + H2O
Carbon dioxide) + Calcium hydroxide → calcium
carbonate + water
Here, Carbon dioxide is a non-metallic
oxide and Calcium hydroxide is a base.
(iii) (a) Reaction of Metal with Acids:
- When metals react with acids they produce a salt and hydrogen gas.
- This reaction is a type of single replacement reaction where the metal displaces the hydrogen in the acid.
- This property is used in industrial processes like remove surface impurities from metals.
- The general form of this equation is:
Metal + Acid → Salt + Hydrogen Gas
Example (i):
Zn + 2HCl → ZnCl2 + H2 (↑)
Zinc + hydrochloric acid → zinc chloride + hydrogen
gas
Example (ii):
Fe + H2SO4 → FeSO4
+ H2 (↑)
iron + sulfuric acid → iron sulfate + hydrogen gas
Example (iii):
2Al + 6HCl → 2AlCl3 + 3H2 (↑)
Aluminum + hydrochloric acid → aluminum chloride + hydrogen
gas
Example (iv):
Mg + 2HNO3 → Mg(NO3)2 + H2 (↑)
Magnesium + nitric acid → magnesium nitrate + hydrogen
gas
Example (v):
2HCl + Mg → MgCl2 + H2 (↑)
Hydrochloric acid + Magnesium → Magnesium chloride
+ Hydrogen
Example (vi):
Zn (s) + H2SO4 (aq) → ZnSO4
(aq) + H2 (g)
Zinc + Sulfuric acid → zinc sulphate + hydrogen gas
(b) Reaction of Metal with Base:
- When metals react with base they produce a salt and hydrogen gas.
- The metal reacts with the base, which donates hydroxide ions (OH—) to the metal.
- The general form of this equation is:
Base + Metal
→ Salt + Hydrogen Gas
Example (i):
2NaOH(aq) + Zn(s) → Na2ZnO2(s)
+ H2(g)
Sodium hydroxide + Zink → Sodium zincate + Hydrogen
gas
Example (ii):
Mg(OH)2 + 2Na → 2NaOH + Mg
Magnesium hydroxide
+ Sodium → Sodium hydroxide + Magnesium
(iv) (a) Reaction of Metal Carbonates
with Acids:
- When metal carbonates react with acids they produce a salt, carbon dioxide gas and water.
- This is a double replacement reaction in which the metal carbonate (a type of base) reacts with the acid and forms a salt, water and carbon dioxide gas.
- The general form of this equation is:
Metal Carbonate + Acid → Salt + Carbon Dioxide +
Water
Example (i):
CaCO3 + 2HCl → CaCl2 + CO2
+ H2O
Calcium carbonate + hydrochloric acid → calcium
chloride + carbon dioxide + water
Example (ii):
MgCO3 + H2SO4 →
MgSO4 + CO2 + H2O
Magnesium carbonate) + sulfuric acid → magnesium
sulfate + carbon dioxide + water
Example (iii):
Na2CO3(s) + 2HCl(aq) → 2NaCl
(aq) + H2O (l) + CO2(g)
Sodium carbonate + Hydrochloric Acid → Sodium
Chloride + water + Carbon dioxide gas
Note: When Carbon dioxide gas passes
through Lime water (Calcium hydroxide solution, CaCO3) it makes
white precipitate.
Ca(OH)2(aq) + CO2(g) → CaCO3
(s) + H2O (l)
(b) Reaction of Metal Carbonates with base:
- When metal carbonates react with bases they produce a salt, carbon dioxide gas and water.
- The general form of this equation is:
Metal Carbonate + Base → Salt + Water +
Carbon Dioxide
Example (i):
2Na2CO3 + 2NaOH → 3Na2CO3
+ H2O + CO2
Sodium carbonate + sodium hydroxide →
sodium carbonate (a salt) + water + carbon dioxide
(v) (a)Reaction of Metal
Hydrogencarbonates with Acids:
- When metal hydrogencarbonates, also known as metal bicarbonates, react with acids they produce a salt, carbon dioxide gas and water.
- This is a double replacement reaction in which the metal hydrogencarbonates (a type of base) reacts with the acid and forms a salt, water and carbon dioxide gas.
- The general form of this equation is:
Metal hydrogencarbonates/bicarbonates + Acid → Salt
+ Carbon Dioxide + Water
Example (i):
NaHCO3(s) + HCl(aq) → NaCl (aq) + H2O
(l) + CO2(g)
Sodium bicarbonate + Hydrochloric Acid → Sodium
Chloride + water + Carbon dioxide gas
Example (ii):
2KHCO3 + H2SO4 → K2SO4
+ 2CO2 + 2H2O
Potassium hydrogencarbonate + sulfuric
acid → potassium sulfate + carbon dioxide + water
Example (iii):
NH4HCO3 + CH3COOH → NH4CH3COO + CO2 + H2O
Ammonium bicarbonate + acetic acid → ammonium
acetate + carbon dioxide + water
(b) Reaction of Metal
Hydrogencarbonates with Base:
- The general form of this equation is:
- Metal Hydrogencarbonate + Base → Salt + Water + Carbon Dioxide
Example (i):
NaHCO3 + NaOH → Na2CO3 + H2O + CO2
Sodium hydrogencarbonate + sodium hydroxide → sodium
carbonate + water + carbon dioxide
Arrhenius’ Theory of Acids and Bases:
According to Arrhenius' theory:
Acids:
- An acid is a substance that, when dissolved in water, releases hydrogen ions (H+) into the solution.
- Acids increase the concentration of H+ ions in an aqueous solution.
- Example is hydrochloric acid (HCl), which dissociates in water to form H+ and Cl— ions:
HCl (aq) → H+ (aq) + Cl— (aq)
Bases:
- A base is a substance that, when dissolved in water, releases hydroxide ions (OH—) into the solution.
- Bases increase the concentration of OH— ions in an aqueous solution.
- Example is sodium hydroxide (NaOH), which dissociates in water to form Na+ and OH— ions:
NaOH (aq) → Na+ (aq) + OH— (aq)
Reactions:
- Acid-base reactions in aqueous solutions result in the formation of water and a salt.
- H+ ions from the acid combine with OH— ions from the base to produce water.
- The remaining ions combine to form a salt.
Bronsted-Lowry theory:
In the Bronsted-Lowry theory:
Acids:
- An acid is a substance that can donate a proton (H+) to another substance in a chemical reaction.
- It acts as a proton (H+) donor.
Bases:
- A base is a substance that can accept a proton (H+) from another substance in a chemical reaction.
- It acts as a proton (H+) acceptor.
Important point in this theory:
- Acids and bases do not need to be in aqueous solutions; the theory applies to reactions in various solvents.
- The theory is not limited to the presence of water, as it focuses on proton transfer.
- According to this theory, water itself can act as both an acid (proton donor) and a base (proton acceptor), making it an amphoteric substance.
- Conjugate acid-base pairs are formed during reactions. An acid's conjugate base is the substance remaining after it donates a proton and a base's conjugate acid is the substance formed after it accepts a proton.
Physical Test of Acid and Base:
Taste:
- Acids have a sour taste, while bases taste bitter.
- However, using the taste method to identify acids or bases is not recommended due to safety concerns. Acids and bases can be corrosive and potentially harmful if ingested.
- For example: foods like curd, lemon juice and vinegar taste sour because they contain acids, while baking soda has a bitter taste which is indicating its basic nature.
Effect on Indicators by Acids and
Bases:
Indicators are chemical substances that change their physical properties, such as color or odor, when exposed to acids or bases. Commonly used indicators and the colors they exhibit are as follows:
(a) Litmus:
- In a neutral solution: Purple
- In an acidic solution: Red
- In a basic solution: Blue
- Litmus is available in two forms: red litmus and blue litmus.
- An acid turns moist blue litmus paper red, while a base turns moist red litmus paper blue.
(b) Methyl Orange:
- In a neutral solution: Orange
- In an acidic solution: Red
- In a basic solution: Yellow
(c) Phenolphthalein:
- In a neutral solution: Colorless
- In an acidic solution: Remains colorless
- In a basic solution: Pink
These indicators are valuable tools for
chemists to determine whether a substance is acidic or basic based on the
observed color changes. They provide a safer and more reliable method than
relying on taste.
Common Characteristics of Acids and
Bases:
Corrosive Nature: Many strong acids and bases share a corrosive
property, which can lead to the degradation or rusting of metals.
Litmus Paper Reaction: Both acids and bases cause distinct color changes
in litmus paper. Acids turn blue litmus paper red, while bases turn red litmus
paper blue.
Skin Hazards: Strong acids can cause severe burns and damage to
the skin upon contact. Interestingly, strong bases can also produce similar
harmful effects on the skin.
Everyday Use: Acids and bases are found in everyday items. For example-
citric fruits like lemons and oranges contain citric acid, while tamarind and grapes
contain tartaric acid. Common household products, such as olive oil and
vinegar, contain oleic acid and acetic acid, respectively. Bases are also found
in various products like soaps and toothpaste, with baking soda being an
example of a basic substance.
Classification Basis: Both acids and bases can be categorized based on
their strength, concentration, basicity or acidity. Acids are also classified
according to their source and the presence of oxygen.
Interaction with Water: Acids and bases are both reactive with water, and
many of them are soluble in aqueous solutions.
Electrolytic Properties: Both acids and bases are considered electrolytes,
meaning they conduct electricity effectively.
Ion Production: Acids release hydrogen ions (H+), while
bases release hydroxide ions (OH—) when dissolved in water.
Exothermic Reaction: When acids or bases are mixed with water, the
process is exothermic, meaning it releases a certain amount of heat.
When an acid is dissolved in water:
Following changes happens-
- Dissolution: When an acid is added to water, it dissolves to
form ions in the solution. The acid molecules break apart into their
constituent ions.
- Ionization: Acidic compounds release hydrogen ions (H+)
into the water. For example, hydrochloric acid (HCl) ionizes to form H+
and Cl— ions:
Hydrogen ions in HCl are produced in the presence of water. The separation of H+ ion from HCl molecules cannot occur in the absence of water.
Hydrogen ions cannot exist alone, but they exist after combining with water molecules. Thus hydrogen ions must always be shown as H+(aq) or hydronium ion (H3O+).
- Increase in H+
Concentration: The addition of the acid increases the
concentration of H+ ions in the water, making the solution more
acidic.
- pH Decrease: As the concentration of H+ ions
increases, the pH of the solution decreases, indicating greater acidity.
- Taste and Color: Acids often have a sour taste and can change the
color of indicators, turning blue litmus paper to red.
When an base is dissolved in water:
Following changes happens-
Dissolution: When a base is added to water, it also dissolves
and breaks into its constituent ions.
Ionization: Bases release hydroxide ions (OH—) into
the water. For example, sodium hydroxide (NaOH) ionizes to form Na+
and OH— ions:
Bases generate hydroxide (OH–) ions in water. Bases which are soluble in water are called alkalis.
Increase in OH—
Concentration: The addition of the base increases the
concentration of OH— ions in the water, making the solution more
basic.
pH Increase: As the concentration of OH— ions
increases, the pH of the solution rises, indicating greater basicity.
Taste and Feel: Bases often have a bitter taste and can feel
slippery or soapy to the touch. They can also change the color of indicators,
turning red litmus paper to blue.
So, we can identified that all acids
generate H+(aq) and all bases generate OH–(aq), we can write
the neutralisation reaction as follows –
Difference between a Base and an Alkali:
|
Base |
Alkali |
|
Bases
engage in neutralization reactions when they react with acids. |
An
alkali refers to an aqueous solution of a base, primarily consisting of
metallic hydroxides. |
|
They
are typically composed of substances such as metal oxides, metal hydroxides,
metal carbonates, and metal bicarbonates. |
When dissolved in water, alkalis
dissociate to release hydroxide ions (OH−). |
|
Many
bases are insoluble in water, which means they do not readily dissolve. |
It's
important to note that all alkalis are bases, but not all bases are alkalis.
Alkalis are a specific subset of bases that are in the form of aqueous
solutions of metallic hydroxides. |
Dilution of Acids and Bases:
- When an acid or base is mixed with water, it leads to a reduction in the concentration of ions, specifically H3O+ ions for acids and OH− ions for bases, per unit volume.
- This reduction in ion concentration is known as dilution.
- Dilution is a process where the original concentration of an acid or base in a solution is lowered by adding more solvent, mostly water.
- As a result of dilution, the solution becomes less concentrated and less acidic or less basic.
- Dilution is often used to prepare solutions with specific concentrations for various scientific and practical applications.
Hydronium Ion:
- A hydronium ion (H3O+) is a positively charged ion that consists of a water molecule (H2O) with an additional hydrogen ion (H+) attached to it.
- In an aqueous solution, when an acid dissolves in water, it donates a proton (H+ ion) to a water molecule, resulting in the formation of a hydronium ion.
- This process is responsible for the characteristic acidity of solutions with a higher concentration of H3O+ ions.
- Hydronium ions play a important role in defining the pH of a solution.
- In acidic solutions, the concentration of H3O+ ions is higher, which corresponds to a lower pH value.
- In neutral solutions, the concentration of H3O+ and OH— ions is equal, resulting in a pH of 7.
- In basic (alkaline) solutions, the concentration of OH— ions is higher, leading to a higher pH value.
Use of Acid-Base Indicators:
Acid-base indicators help distinguish
between acids and bases based on color changes.
Quantifying Hydrogen (H+) and Hydroxide
(OH-) Ions:
- The concentration of H+ and OH- ions in a solution can be quantitatively determined.
- The strength of an acid or base can be judged by the amount of H+ or OH- ions it produces.
Universal Indicator:
- A universal indicator is a mixture of several indicators that changes color at different hydrogen ion concentrations.
- It allows for a broader pH range measurement.
pH Scale:
- The pH scale measures the hydrogen ion concentration in a solution.
- pH values range from 0 (very acidic) to 14 (very alkaline).
- The pH of a neutral solution is 7.
- Values less than 7 indicate acidity, while values from 7 to 14 indicate alkalinity.
Measurement of pH:
- Universal indicator-impregnated paper is commonly used to measure pH.
- The pH of a solution represents its acidic or basic nature.
Strength of Acids and Bases:
- The strength of acids depends on the number of H+ ions they produce.
- Strong acids release more H+ ions, while weak
acids release fewer H+ ions.
- Strong bases release more OH— ions, while weak bases release fewer OH— ions.
- Dilute acid: contains less number of H+(aq) ions per
unit volume.
- Concentrated acid: contains more number of H+(aq) ions per
unit volume.
Strong vs. Weak Bases:
- Strong bases release a higher concentration of OH—
ions and are considered strong.
- Weak bases release a lower concentration of OH—
ions and are considered weak.
Importance of pH in Everyday Life:
pH Sensitivity of Living Organisms:
- Living organisms, including plants and animals, are sensitive to pH.
- The human body functions within a narrow pH range of 7.0 to 7.8.
Acid Rain Effects:
- Rainwater with a pH below 5.6 is termed "acid rain."
- Acid rain can lower the pH of rivers, making it challenging for aquatic life to survive.
pH in Soil and Plant Growth:
- Plants require specific pH levels for healthy growth.
- The pH of soil can be determined to assess its suitability for particular plant growth.
- The pH of a soil optimal for the growth of plants or crops is 6.5 to 7.0.
pH in Digestive System:
- The stomach produces hydrochloric acid for digestion.
- Excessive acid production during indigestion leads to discomfort.
- The process of digestion happens at a specific pH in our stomach which is 1.5 to 4.
- Antacids, such as magnesium hydroxide (Milk of Magnesia), neutralize excess stomach acid.
Tooth Decay Prevention:
- Tooth decay occurs when the mouth's pH drops below 5.5.
- Tooth enamel, composed of calcium hydroxyapatite, is corroded at low pH.
- Bacteria in the mouth produce acids from sugar and food particles, contributing to tooth decay.
- Regular oral hygiene, using basic toothpaste, can neutralize excess acid and prevent tooth decay.
Chemical Defense Mechanisms in Animals
and Plants:
- Some organisms, like bees, employ chemical warfare for self-defense.
- Bee stings leave an acid that causes pain and irritation.
- Applying a mild base, such as baking soda, to the stung area provides relief.
- Nettle leaves inject methanoic acid when touched, causing a burning pain.
Some acids provided by nature:
|
Natural source |
Acid |
Natural source |
Acid |
|
Vinegar |
Acetic acid |
Sour milk (Curd) |
Lactic acid |
|
Orange |
Citric acid |
Lemon |
Citric acid |
|
Tamarind |
Tartaric acid |
Ant sting |
Methanoic acid |
|
Tomato |
Oxalic acid |
Nettle sting |
Methanoic acid |
Salts:
- Salt is a combination of an anion of an acid and a cation of a base.
- Salts are usually prepared by the neutralisation reaction of an acid and a base.
- Salts have the same positive or negative radicals.
Salts of a Strong Acid and Strong Base:
- Result in a neutral solution with a pH value of 7.
- Neither acidic nor basic; pH is neutral.
Salts of a Strong Acid and Weak Base:
- Result in an acidic solution with a pH value below 7.
- The presence of excess H+ ions causes acidity.
Salts of a Strong Base and Weak Acid:
- Produce a basic solution with a pH value greater than 7.
- The excess OH- ions contribute to basicity.
Chemical formulae of some common salts:
- Potassium sulfate: K2SO4
- Sodium sulfate: Na2SO4
- Calcium sulfate: CaSO4
- Magnesium sulfate: MgSO4
- Copper sulfate: CuSO4
- Sodium chloride: NaCl
- Sodium nitrate: NaNO3
- Sodium carbonate: Na2CO3
- Ammonium chloride: NH4Cl
- Potassium nitrate: KNO3
- Aluminum chloride: AlCl3
- Zinc sulphate: ZnSO4
- Sodium acetate: CH3COONa
- Sodium hydrogencarbonate: NaHCO3
Chemicals from Common Salt:
- The salt formed by combining hydrochloric acid and sodium hydroxide solution is known as sodium chloride (NaCl), commonly used in food. It is a neutral salt.
- Seawater contains various dissolved salts, and sodium chloride is one of them. It can be separated from seawater. It is often brown due to impurities, are found in different parts of the world, known as rock salt.
- Rock salt was formed when ancient seas dried up, and it is extracted through mining methods similar to coal mining.
Common salt — A raw material for
chemicals
- Common salt is a valuable raw material for various everyday products, including sodium hydroxide, baking soda, washing soda, bleaching powder and more.
- It is used in our meals as a flavour enhancer as well as a preservative.
By common salt following four compounds
may be makes:
- Sodium hydroxide or lye or caustic soda
- Baking soda or sodium hydrogen carbonate or sodium bicarbonate
- Washing soda or sodium carbonate decahydrate
- Bleaching powder or calcium hypochlorite
Sodium hydroxide:
- It is a highly versatile and caustic inorganic compound known by several other names, including caustic soda and lye.
- Sodium hydroxide is a strong base and it is highly alkaline.
- It is a white, odorless and highly hygroscopic solid at room temperature.
- It is used in Chemical Industry, Pulp and Paper Industry, Soap and Detergent Manufacturing, Water Treatment, Food Industry and Pharmaceuticals.
Preparation of Sodium Hydroxide:
- Sodium hydroxide is commonly produced through the chlor-alkali process.
- The process is named for its products: "chlor" for chlorine and "alkali" for sodium hydroxide.
- The chlor-alkali process is an industrial method that uses electricity to break down saltwater (brine) into three valuable products: chlorine gas, sodium hydroxide (caustic soda) and hydrogen gas.
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g)
+ H2(g)
- In this process:
- Chlorine gas (Cl2) is produced at the anode.
- The anode is the electrode where oxidation occurs.
- Hydrogen gas (H2) is produced at the cathode.
- Sodium hydroxide solution (NaOH) is formed near the cathode.
- The cathode is the electrode where reduction takes place.
- At last, Sodium hydroxide remains in the solution.
Bleaching powder:
- Bleaching powder is produced using chlorine gas obtained from the electrolysis of aqueous sodium chloride (brine).
- The production involves the reaction of chlorine gas with dry slaked lime [Ca(OH)2].
Ca(OH)2 + Cl2 → CaOCl2
+ H2O
- Bleaching powder is often represented as CaOCl2, although its actual composition is complex.
Uses of Bleaching powder:
- Bleaching powder is used in the textile industry for bleaching cotton and linen fabrics.
- It is used in paper factories to bleach wood pulp, which is a crucial step in paper production.
- It is used in laundry to bleach washed clothes, removing stains and restoring whiteness.
- Bleaching powder used as an oxidizing agent in various chemical industries.
- It participates in chemical reactions where oxidation is required for the synthesis of various compounds.
- It is used to disinfect and make drinking water free from harmful germs.
- Bleaching powder's germicidal properties help ensure safe and clean drinking water.
Baking soda (Sodium Hydrogencarbonate -
NaHCO3):
- Often used in the kitchen for culinary purposes, such as making crispy pakoras and speeding up the cooking process.
- Baking soda can be used to neutralize acids due to its mild, non-corrosive basic properties.
- Baking soda is produced using sodium chloride (NaCl) as one of the raw materials. In this process, CO2 is passed through a concentrated solution of sodium chloride and ammonia. This process is known as Solvay process.
NaCl(aq) + H2O(l) + CO2(g) +
NH3(g) → NH4Cl(aq) + NaHCO3(aq)
- When heated during cooking, baking soda undergoes a reaction to produce sodium carbonate (sodium salt of an acid), carbon dioxide (CO2) and water (H2O).
Uses of Backing Soda:
- Baking soda is a mixture of baking soda and a mild edible acid like tartaric acid.
- When baking powder is heated or mixed in water, it generates carbon dioxide (CO2), causing baked goods like bread or cakes to rise and become soft and spongy.
NaHCO3
+ H+ → CO2 + H2O + Sodium salt of acid
- Baking soda is used as an ingredient in antacids.
- Its alkaline nature enables it to neutralize excess acid in the stomach, providing relief from acidity and heartburn.
- Baking soda is used in soda-acid fire extinguishers.
- When the extinguisher is activated, it reacts with an acid to release carbon dioxide (CO2), which helps suppress fires by smothering them.
Washing soda (Sodium Carbonate - Na2CO3.10H2O):
- It can be obtained by heating baking soda (sodium hydrogencarbonate) and through recrystallization of sodium carbonate.
Na2CO3
+ 10H2O → Na2CO3.10H2O
- The formula Na2CO3.10H2O signifies that washing soda contains 10 water molecules (H2O) per molecule of sodium carbonate. This makes washing soda a hydrated compound but it doesn't mean that it is wet.
Uses of Washing Soda:
- Sodium carbonate is used in the glass, soap and paper industries for various purpose.
- It is used for manufacture of other sodium compounds, such as borax (sodium borate).
- Sodium carbonate can be used as a cleaning agent for domestic purposes, like- cleaning surfaces and laundry.
- It is used for removing permanent hardness of water, making it suitable for various industrial and household applications.
Are the Crystals of Salts really Dry?
- Water of crystallization refers to a fixed number of water molecules that are part of the crystal structure of certain salts.
- When copper sulfate crystals, which contain water of crystallization, are heated, the water is removed, and the salt turns white. This can be represented as the dehydration reaction:
CuSO4.5H2O(s)
→ CuSO4(s) + 5H2O(g)
- Re-moistening these crystals with water restores the blue color, as the water of crystallization is reabsorbed. This can be represented as the rehydration reaction:
CuSO4(s)
+ 5H2O(g) → CuSO4.5H2O(s)
- Copper sulfate (CuSO4) has five water molecules as water of crystallization in one formula unit, giving it the chemical formula CuSO4.5H2O.
- Another example is : Gypsum (CaSO4) has two water molecules as water of crystallization, with the chemical formula CaSO4.2H2O.
Plaster of Paris (calcium sulphate hemihydrates,
CaSO4.½H2O):
- Plaster of Paris is a white powder.
- Plaster of Paris is formed by heating gypsum (CaSO4.2H2O) at 373 K (100°C).
- During this process, gypsum loses some of its water molecules and transforms into calcium sulfate hemihydrate, which has the chemical formula CaSO4.½H2O.
- When we mixed it with water it changes back into gypsum and form a hard and solid mass.
CaSO4.½H2O
+1½H2O → CaSO4.2H2O
- Only half a water molecule is shown to be attached as water of crystallization because two formula units of CaSO4 share one molecule of water. So, it's called "hemihydrate".
Uses of Plaster of Peris:
- Plaster of Paris is widely used in the medical field for making casts and molds to support fractured bones and maintain them in the correct position during the healing process.
- It is also used in making toys, decorative materials and creating smooth surfaces on walls and ceilings.

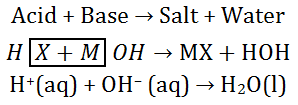



No comments:
Post a Comment
If you have any questions or uncertainties, please don't hesitate to ask.